Welcome to Bodhiclasses – your friendly space for mastering science through clarity, creativity, and curiosity. Today, we’re diving deep into NCERT Class 7 Science Chapter 5 – Changes Around Us: Physical and Chemical with detailed, well-explained answers to all in-text and end-of-chapter questions. Whether you’re a student revising for exams or a teacher looking for ready classroom material, this is your go-to resource.

1. Which of the following statements are the characteristics of a physical change?
(i) The state of the substance may or may not change.
(ii) A substance with different properties is formed.
(iii) No new substance is formed.
(iv) The substance undergoes a chemical reaction.
Correct answer: ✅ (c) (i) and (iii)
Explanation: A physical change may involve change in state, size, or shape, but no new substance is formed.
2. Predict which of the following changes can be reversed and which cannot be reversed.
| Change | Reversible / Irreversible | Reason |
|---|---|---|
| (i) Stitching cloth to a shirt | ❌ Cannot be reversed | Stitching alters the shape permanently. |
| (ii) Twisting of straight string | ✅ Can be reversed | The string can be straightened again. |
| (iii) Making idlis from a batter | ❌ Cannot be reversed | Chemical change during steaming. |
| (iv) Dissolving sugar in water | ✅ Can be reversed | Sugar can be recovered by evaporation. |
| (v) Drawing water from a well | ✅ Can be reversed | Water can be put back. |
| (vi) Ripening of fruits | ❌ Cannot be reversed | New substances are formed in ripening. |
| (vii) Boiling water in an open pan | ✅ Can be reversed | Vapour can be condensed back. |
| (viii) Rolling up a mat | ✅ Can be reversed | No new substance formed. |
| (ix) Grinding wheat grains to flour | ❌ Cannot be reversed | Original grains can’t be restored. |
| (x) Forming of soil from rocks | ❌ Cannot be reversed | Happens over years, irreversible weathering. |
3. True or False
| Statement | Answer | Correct Statement (if False) |
|---|---|---|
| (i) Melting of wax is necessary for burning a candle. | ✅ True | — |
| (ii) Collecting water vapour by condensing involves a chemical change. | ❌ False | It is a physical change. |
| (iii) The process of converting leaves into compost is a chemical change. | ✅ True | — |
| (iv) Mixing baking soda with lemon juice is a chemical change. | ✅ True | — |
4. Fill in the blanks
(i) Nalini observed that the handle of her cycle has got brown deposits. The brown deposits are due to rusting, and this is a chemical change.
(ii) Folding a handkerchief is a physical change and can be reversed.
(iii) A chemical process in which a substance reacts with oxygen with evolution of heat is called combustion, and this is a chemical change.
(iv) Magnesium, when burnt in air, produces a substance called magnesium oxide. The substance formed is basic in nature. Burning of magnesium is a chemical change.
5. Are the changes of water to ice and water to steam physical or chemical? Explain.
Answer:
They are physical changes because no new substance is formed. Water simply changes its state (solid, liquid, gas), but its chemical composition (H₂O) remains the same.
6. Is curdling of milk a physical or chemical change? Justify your statement.
Answer:
Curdling of milk is a chemical change. A new substance (curd) is formed with different taste and properties. It cannot be reversed back into milk.
7. Natural factors, such as wind, rain, etc., help in the formation of soil from rocks. Is this change physical or chemical and why?
Answer:
It involves both physical and chemical changes:
- Physical: Breaking of rocks due to wind, temperature, and pressure.
- Chemical: Reaction of water and air with minerals in rocks forming new substances (e.g., iron oxide).
8. Eco-friendly Prithvi – Select the correct type of changes
Suggested Title: Prithvi’s Kitchen Science Journey
| Activity | Type of Change |
|---|---|
| Chopping vegetables, peeling potatoes, cutting fruits | ✅ Physical changes |
| Collecting seeds, peels in a pot | ✅ Physical change |
| Decomposing into compost | ✅ Chemical change |
| Germination and blooming | ✅ Chemical change |
9. Classify the changes as Physical (A), Chemical (B), or Both (C): Process of burning a candle; Tearing of paper; Rusting; Curdling of milk; Ripening of fruits; Melting of ice; Folding of clothes; Burning of magnesium and Mixing baking soda with vinegar.
| A (Physical) | B (Chemical) | C (Both) |
|---|---|---|
| Tearing of paper | Rusting | Burning a candle |
| Melting of ice | Curdling of milk | |
| Folding of clothes | Ripening of fruits | |
| Burning of magnesium | ||
| Mixing baking soda with vinegar |
10. Lime water experiment – Which cases turn lime water milky and why?
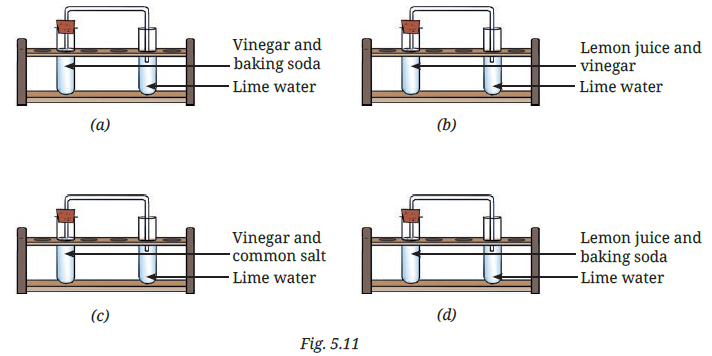
| Setup | Result | Reason |
|---|---|---|
| (a) Vinegar + baking soda | ✅ Lime water turns milky | CO₂ gas formed |
| (b) Vinegar + common salt | ❌ No reaction | No CO₂ |
| (c) Lemon juice + vinegar | ❌ No reaction | No CO₂ |
| (d) Lemon juice + baking soda | ✅ Lime water turns milky | CO₂ gas formed |
Explanation:
CO₂ gas reacts with lime water to form calcium carbonate, which is insoluble, making the lime water appear milky.


