At Bodhiclasses, we believe that learning is not just about acquiring information—it’s about awakening the mind. Rooted in the values of curiosity, clarity, and connection, Bodhiclasses is more than an educational platform; it’s a space where knowledge is nurtured with care and presented with purpose. Here is a complete solution set for the NCERT textbook questions based on Class 7 Science Chapter 2: Exploring Substances – Acidic, Basic, and Neutral.

Q1. A solution turns the red litmus paper to blue. Excess addition of which of the following solutions would reverse the change?
(i) Lime water
(ii) Baking soda
(iii) Vinegar
(iv) Common salt solution
Answer:
(iii) Vinegar
Explanation:
Vinegar is an acidic substance. Since the solution turned red litmus paper to blue, it is basic in nature. To reverse this change (i.e., turn it back to red), an acid must be added. Vinegar, being an acid, neutralizes the base and restores the acidic condition.
Q2. You are provided with three unknown solutions A, B, and C. After testing them using indicators, the following observations were made:
- Solution A turns red litmus to blue → Basic
- Solution B turns turmeric paper red → Basic
- Solution C turns red rose extract green → Basic
Which of the following is the correct sequence for the nature of A, B, and C?
(i) Acidic, acidic, and acidic
(ii) Neutral, basic, and basic
(iii) Basic, basic, and acidic
(iv) Basic, basic, and basic
Answer:
(iv) Basic, basic, and basic
Explanation:
All three observations confirm the basic nature of the solutions:
- Red litmus turning blue (A) indicates a base.
- Turmeric turning red (B) is specific to bases.
- Red rose extract turning green (C) also confirms basicity.
Q3. Label the nature of solutions in Figs. 2.13, 2.14, and 2.15 (red rose extract paper strips used).
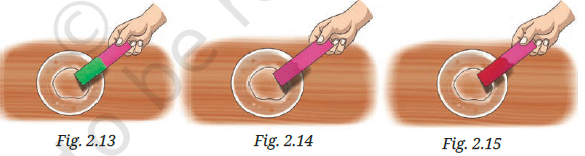
Answer:
Since the red rose extract turns:
- Red in acidic solutions
- Green in basic solutions
- No change in neutral solutions
Assuming:
- Fig. 2.13 shows red colour → Acidic
- Fig. 2.14 shows green colour → Basic
- Fig. 2.15 shows no change → Neutral
Q4. A liquid is tested with red litmus, blue litmus, and turmeric. Observations:

Identify the nature of the liquid and justify.
Answer:
The liquid is acidic in nature.
Justification:
- Blue litmus turning red is a clear sign of acidity.
- No change in red litmus or turmeric confirms it’s not basic.
- Hence, the solution is acidic.
Q5. Manya is blindfolded. She is asked to test two unknown solutions to determine whether they are acidic or basic. Which indicator should she use and why?
Answer:
Manya should use an olfactory indicator, such as onion extract.
Reason:
Olfactory indicators change their smell in acidic or basic environments. Since Manya is blindfolded and cannot rely on visual changes, smell-based indicators are most appropriate for identifying the nature of the solution.
Q6. Suggest materials to write a secret message on white paper and substances to spray for revealing it. Prepare a table.
Answer:
| Writing Material (Basic) | Spray (Indicator/Acidic) | Visible Colour |
|---|---|---|
| Soap solution | Turmeric solution | Reddish-brown writing |
| Baking soda solution | Red rose extract | Green writing |
| Lime water | Purple cabbage extract | Bluish-green writing |
Explanation:
Basic materials remain invisible on paper. Spraying with natural indicators causes color reactions, revealing the message.
Q7. Grape juice mixed with red rose extract turns red. What will happen if baking soda is added? Justify.
Answer:
On adding baking soda (a base), the solution will turn green.
Justification:
Red rose extract is a natural indicator:
- It gives a red tint in acidic media (as with grape juice).
- On adding baking soda, the solution becomes basic, hence the extract turns green.
Q8. Keerthi wrote a message using orange juice. How can her grandmother reveal it?
Answer:
Use turmeric solution as the spray.
Explanation:
Orange juice is slightly acidic. However, to make the writing visible, it should first be on paper that was coated with turmeric. When the message (written using a base like soap) is sprayed with turmeric, reddish-brown text appears.
Q9. How can natural indicators be prepared? Give an example.
Answer:
Natural indicators can be made by extracting colored pigments from plants, flowers, or spices.
Example:
- Turmeric Indicator:
- Mix turmeric powder with water to make a paste.
- Apply to paper and let dry.
- Test with acidic or basic substances.
- Paper turns reddish-brown in bases, no change in acids.
Q10. You are given vinegar, baking soda solution, and sugar solution. Can you identify them using turmeric paper?
Answer:
Yes.
- Baking soda solution turns turmeric paper red → Basic
- Vinegar and sugar solution show no change.
- To differentiate:
- Vinegar is sour and acidic (can be confirmed using blue litmus).
- Sugar solution is neutral.
- To differentiate:
Conclusion: Turmeric paper helps identify only the basic substance—baking soda.
Q11. Red rose extract turns liquid X green. What is the nature of X? What happens on adding amla juice?
Answer:
- Nature of X: Basic (green indicates a base)
- Adding amla juice (acidic) neutralizes X.
- The solution may become neutral or acidic, and the color will shift towards red or no color.
Q12. Complete the following flowchart:
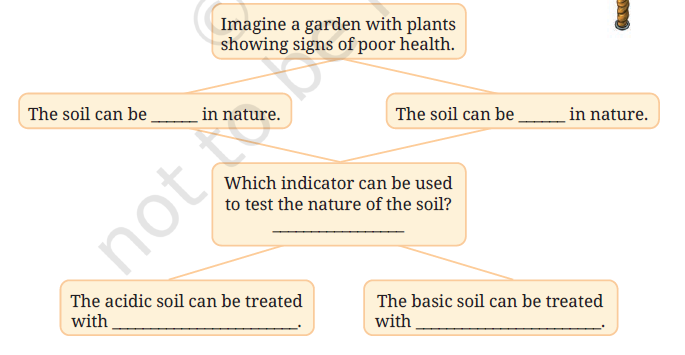
Answer:
Imagine a garden with plants showing signs of poor health.
⬇️
The soil can be acidic in nature. The soil can be basic in nature.
⬇️
Which indicator can be used to test the nature of the soil?
Red cabbage extract / Litmus paper
⬇️
The acidic soil can be treated with lime (calcium carbonate).
The basic soil can be treated with organic matter like compost or manure.

